Immunomodulation in Inflammation and Cancer
Our laboratory is interested in understanding the molecular mechanisms leading to inflammatory diseases and cancer using the mouse as a model system. We investigate the cell-specific role of EGFR signaling in cancer and tumor stromal cells and their complex interaction.
Moreover, we aim to understand how inflammatory myeloid cells affect tissue inflammation and tumor development. We want to exploit new concepts to modulate tumors to become more sensitive to current and novel cancer treatments.
The ultimate goal is to translate this knowledge to patients in order to develop more effective personalized treatments for human cancer.
EGFR signaling in cancer development and inflammation
EGFR overexpression or mutations are present in many human tumors of epithelial and glial origin and targeted anti-EGFR therapies are currently used for cancer treatment. Until recently the tumor-promoting function of the EGFR was exclusively linked with its expression in tumor cells.
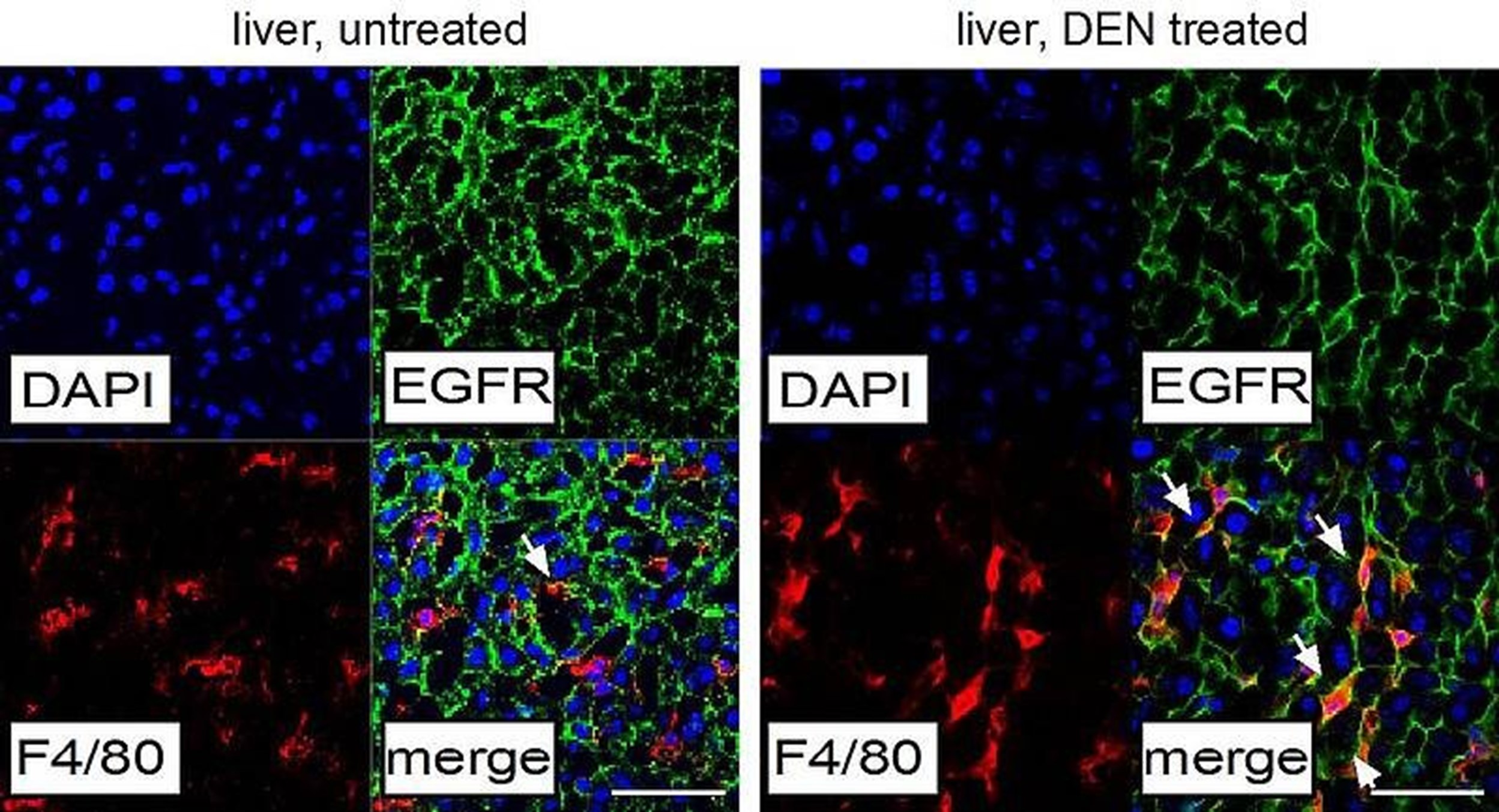
Our laboratory has recently discovered that in liver tumors (HCC) EGFR is upregulated in resident macrophages (also called Kupffer cells) (Figure 1), where it has a tumor-promoting function by regulating the expression of cytokines such as IL-6.By employing genetically engineered mouse models (GEMMs), we demonstrated that deletion of EGFR in macrophages dramatically reduces liver cancer development, whereas EGFR deletion in tumor cells is even accelerating tumor growth.
Also in HCC patients we could demonstrate that the presence of EGFR positive macrophages is a bad prognostic factor for disease-free and overall survival of patients. This tumor-promoting function of EGFR in non-tumor cells is very important for precision oncology as it allows a better stratification and more effective treatment of liver cancer patients for which there are still no therapeutic options (Lanaya et. al. Nature Cell Biology, 2014).
In human CRC we detected EGFR positive myeloid cells in the tumor microenvironment, which correlated with poor survival in metastatic patients. In CRC mouse models we demonstrated that EGFR inhibition in myeloid cells reduces tumor growth whereas therapeutic deletion of EGFR in tumor cells does not. These finding force us to re-evaluate the mechanism by which anti-EGFR drugs are effective in tumors (Srivatsa et al. Gastroenterology. 2017 Jul;153(1):178-190.).
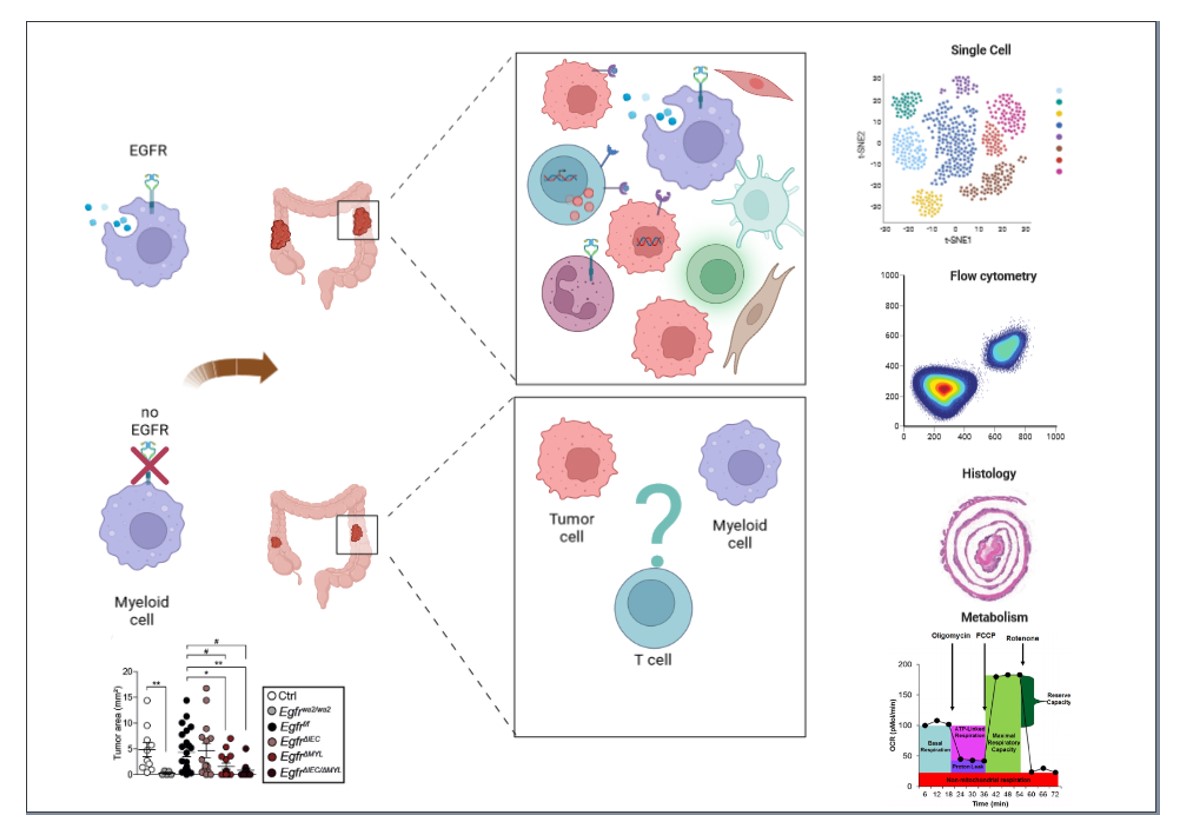
We are now interested in identifying the role of EGFR in specific myeloid cell subpopulations and to characterize inflammatory and metabolic pathways by which EGFR in myeloid cell is regulating interaction with other cells in the tumor microenvironment (Figure 2).
Therapeutic targeting of EGFR in colorectal cancer as a novel approach to predict and enhance tumor immmmunogenicity and response to checkpoint inhibitors
Despite the big success of checkpoint inhibitors for cancer treatment, many patients like microsatellite stable metastatic colorectal cancer (mCRC) patients fail to respond for reasons that are poorly understood. One standard therapy for mCRC, with wildtype RAS, is EGFR inhibition combined with chemotherapy. However, for unknown reasons, many patients do not profit from this therapy.
Using genetically engineered mouse models (GEMM) we identified a tumor-promoting role of EGFR-expressing myeloid cells in CRC and could demonstrate that EGFR positive myeloid cells are a bad prognostic factor for mCRC patients. We thus hypothesize that EGFR-expressing myeloid cells adopt a pro-tumorigenic phenotype by creating an immunosuppressive environment. EGFR blockade could therefore revert this by increasing immunity against tumors and enhancing the effectiveness of checkpoint inhibitors.
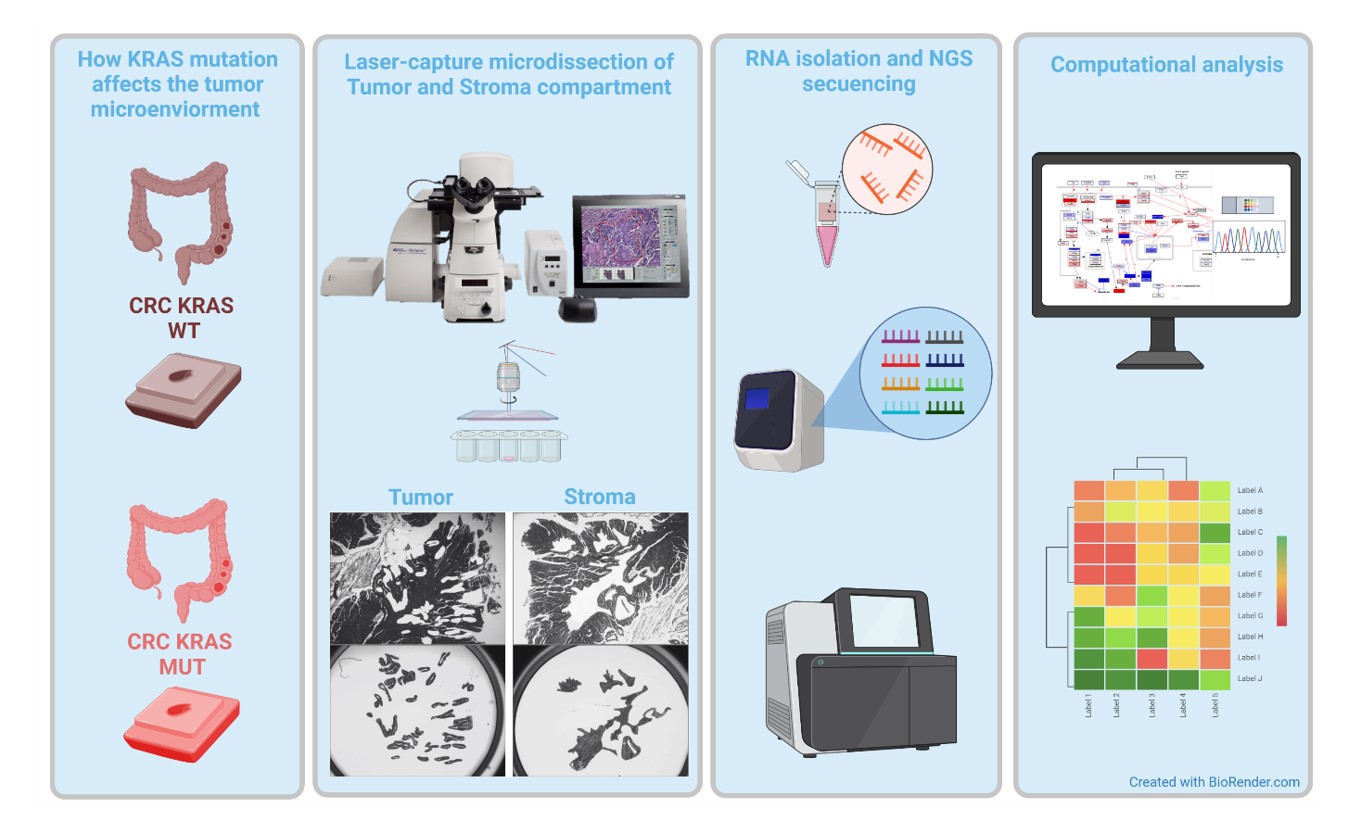
Together with a group of experts in oncology, bioinformatics and molecular biology we are testing this hypothesis by employing GEMM of CRC lacking EGFR in different cells, tumor- or immune cells, combined with mouse and human next generation sequencing of tumor, stromal, and immune cell populations.
The complex interplay between mutations in tumor cells and stroma will be tackled by investigations on large patient cohorts combined with mechanistic studies in GEMM (Figure 3).
We expect to identify factors conferring resistance to immunotherapy in microsatellite stable CRC patients with the aim to improve precision oncology in mCRC. To confirm our findings in pre-clinical animal models we utilize mouse organoids derived from different EGFR mutant strains in combination with immune therapy.
Understanding the side effects caused by anti-EGFR therapy
The majority of cancer patients receiving anti-EGFR therapies also develop a severe skin inflammation, which positively correlates with the anti-cancer treatment response. Mice lacking EGFR in the epidermis develop severe hair follicle defects and skin inflammation similar to what observed in patients receiving anti-EGFR therapies.
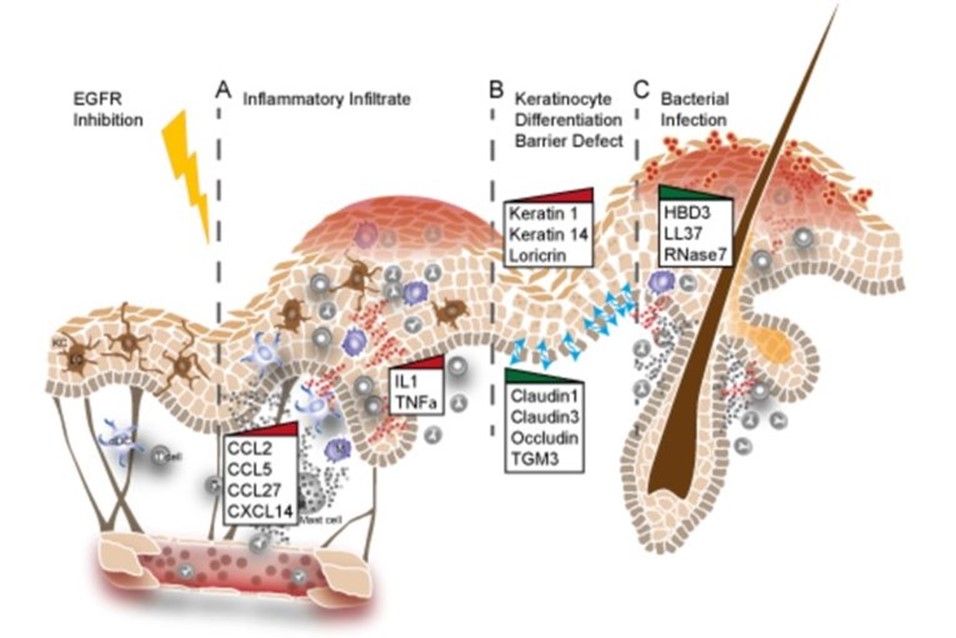
Using GEMMs and patient skin samples we could show that EGFR inhibition leads to the upregulation of inflammatory cytokines and breakdown of the skin barrier (Figure 4). In depth understanding of the molecular mechanisms at the basis of these defects will greatly contribute to develop strategies aimed at alleviating the side effects of patients receiving anti-EGFR drugs (Klufa et al. Sci Transl Med. 2019 Dec 11;11(522), (Lichtenberger, Holcmann, Gerber et al. Sci Transl.Med. 2013 Aug 21;5(199)).
We are now studying the role of different hair follicle cell populations and their influence on the skin microbiome.
Function of dendritic cells in tumor development and skin inflammation
GEMMs are also employed to analyze the role of innate immune cells like plasmacytoid DCs (pDCs) and conventional DC (cDC)in inflammation and cancer with the aim to find therapeutic intervention strategies to enhance innate immunity against tumors.
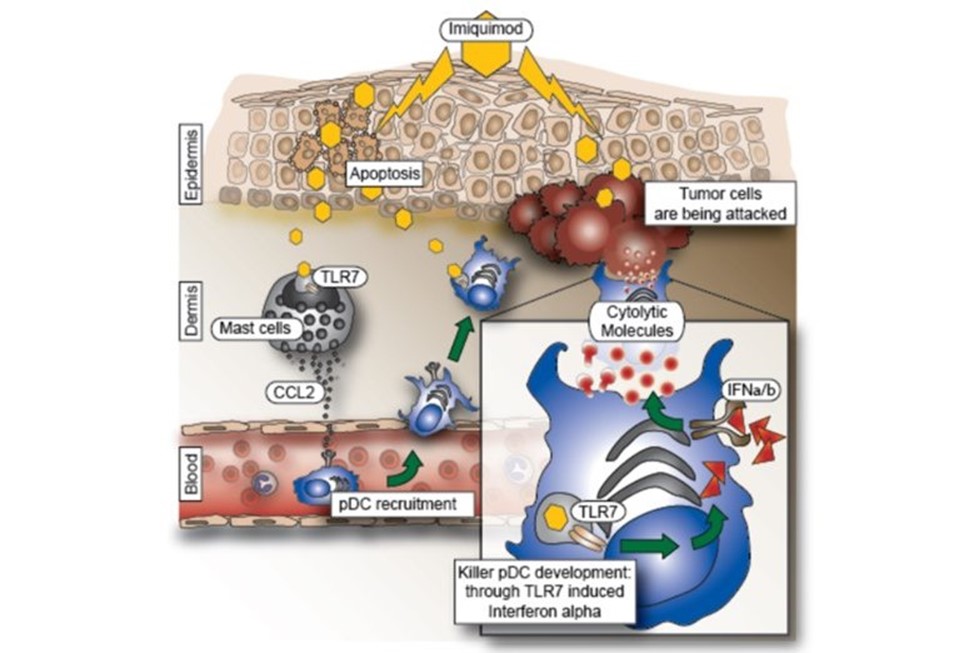
We discovered that if pDC are activated by Imiquimod, a Toll-like receptor (TLR) 7/8 agonist used in the clinic to treat basal cell carcinomas, they can be converted into “killer cells” capable of clearing tumors without the need of the adaptive immune system (Drobits et. al. JCI, 2012). Thus, Imiquimod could serve as a useful adjuvant and immunotherapeutic agent capable of skewing inflammatory responses towards tumor inhibiting and tumor killing responses (Figure 5).
We are currently investigating whether pDC regulate anti-tumor immune responses also in other organs such as the gut and the liver.
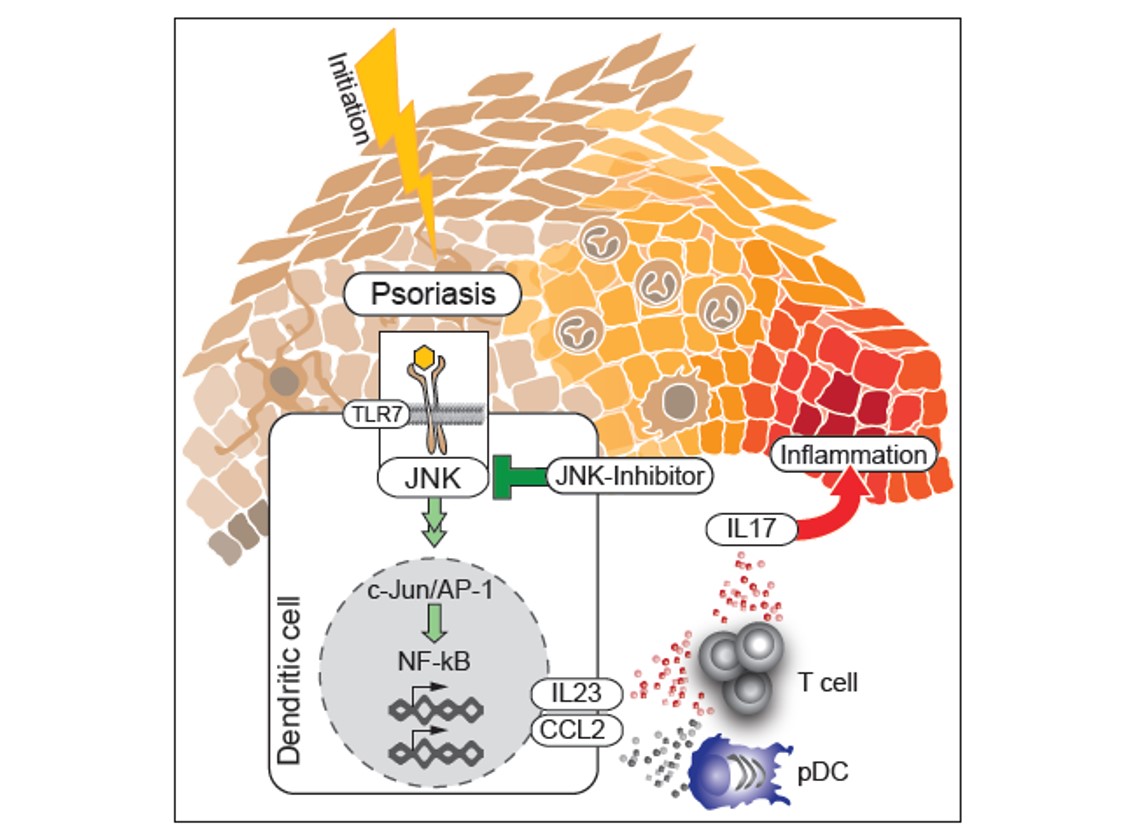
The recruitment of pDC into the skin and the skin inflammation induced by topical treatment with Imiquimod are mediated by CCL2. We could show that cutaneous Type2 DC (cDC2) are the main producers of CCL2 in a c-jun dependent manner. C-jun in cDC2 is also needed for the production of IL23, a key mediator of psoriasis. We found co-expression of c-jun, CCL2 and IL-23 in inflammatory DC in diseased but not healthy skin of psoriasis patients and blockade of AP-1/JNK in human DC reduced expression of CCL2 and IL-23 (Figure 6). Thus, inhibitors of AP-1 are potential novel candidates for the treatment of psoriasis, (Novoszel et al. EMBO Mol.Med. 13, 2021).
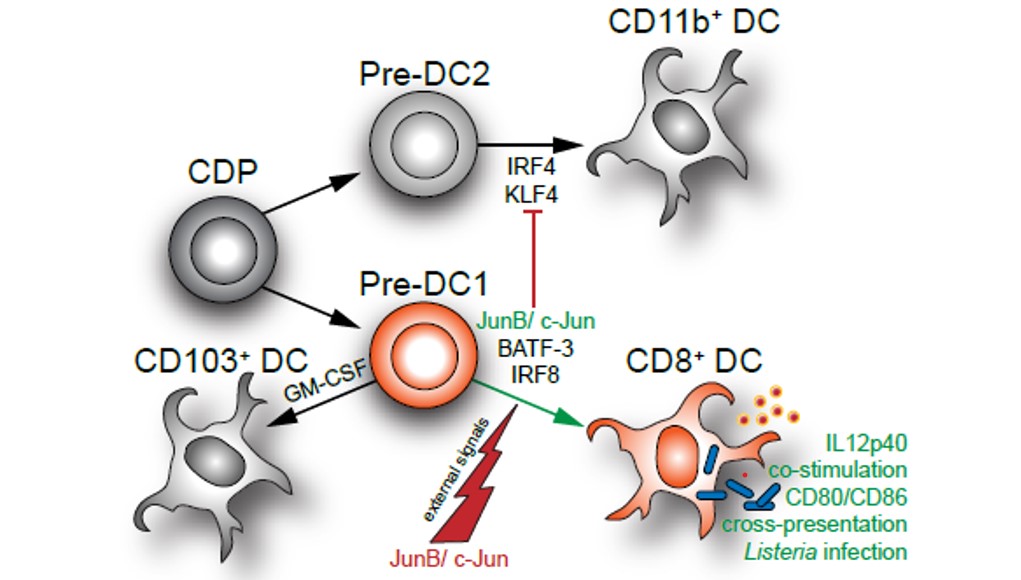
AP-1 transcription factors also regulate differentiation and function of Type1 DC (cDC1). Loss of c-jun and junB in DC progenitors in mice affects diversification of cDC1. In the skin as well as in secondary lymphoid organs CD8a positive cDC1 are reduced and acquire cDC2 properties, whereas CD103 positive migratory cDC1 development is not affected (Figure 7). The defect in CD8a+ cDC1 affects DC functions such as antigen presentation and clearance of bacterial infections (Novoszel et al. Cell Death & Differentiation, 2021). We are therefore currently investigating the role of AP-1 in DC in tumor development.