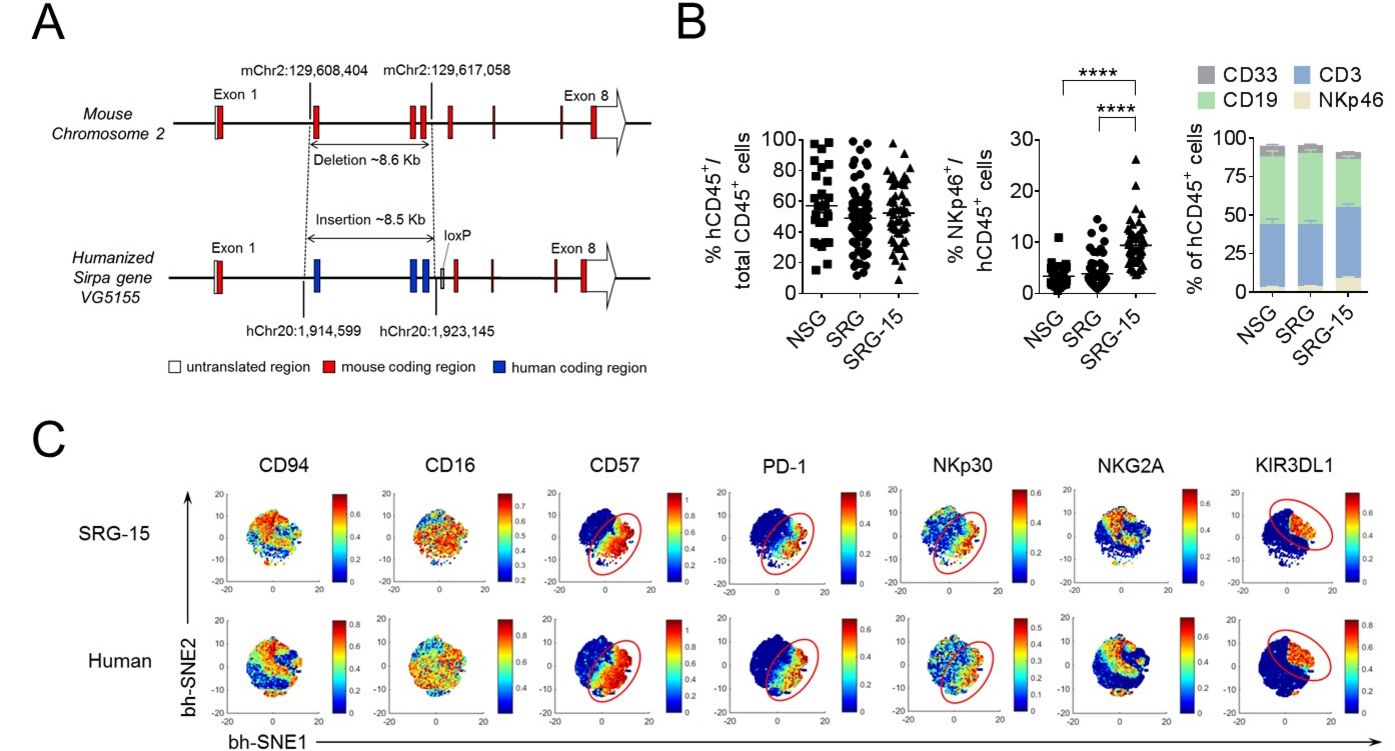
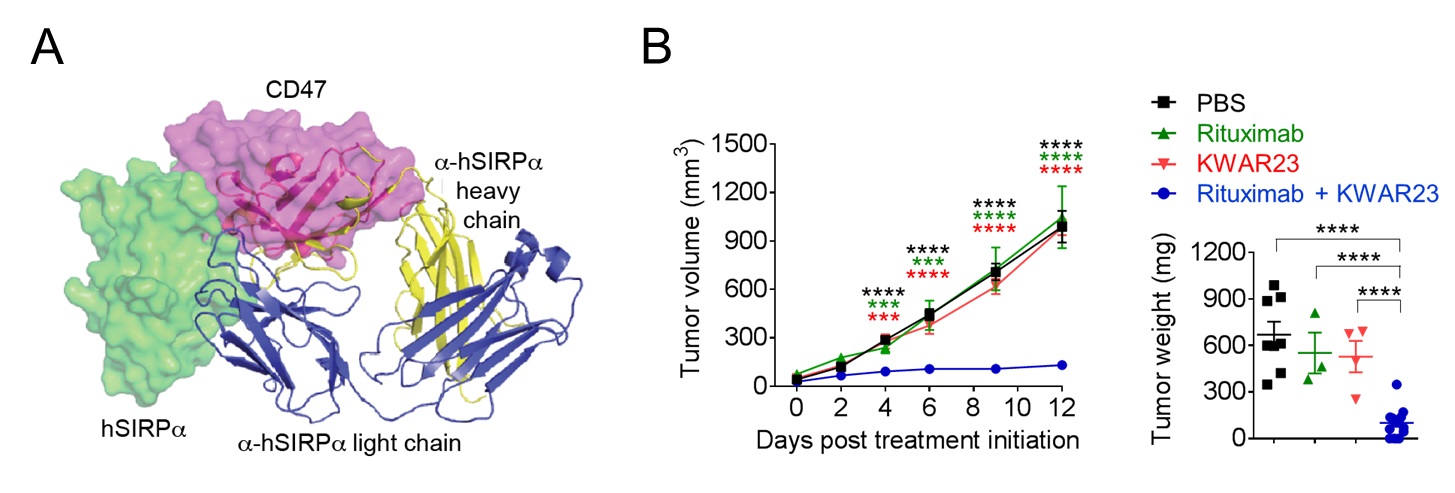
Humanized mouse models for translational cancer research and immunotherapy
Humanized mice (immunodeficient mice reconstituted with a human immune system) represent a promising tool for precision medicine as they may allow modeling and therapy of human diseases in vivo. We use a humanized mouse model that supports the development of a functional human immune system, the growth of patient-derived colorectal cancers, and cancer immunotherapy. Our humanized mouse model will allow us to investigate how the tumor microenvironment regulates human anti-tumor immune responses in vivo, which mechanisms promote immune cell exhaustion, tumor resistance and metastasis, and which combination therapies will be most successful in eliminating specific types of colorectal cancer.
Long noncoding RNA regulation of adaptive immunity
Long noncoding RNAs (lncRNAs) play a key role in regulating tumor growth and immune cell responses. By using deep RNA-seq we identified novel lncRNAs regulating adaptive immunity. By using CRISPR/Cas9-mediated genome engineering, we generated lncRNA knockout mice to understand how they regulate immune cell development and adaptive immunity.
Stromal regulation of immune cell development
Stromal cells maintain hematopoietic stem cells (HSC) and provide lineage-instructive differentiation signals to lymphoid progenitors. We have developed reporter and conditional knockout mice to investigate the role of distinct subsets of stromal cells for immune cell development and the regulation of immune cell responses.