Juliane Winkler, PhD
Research Focus
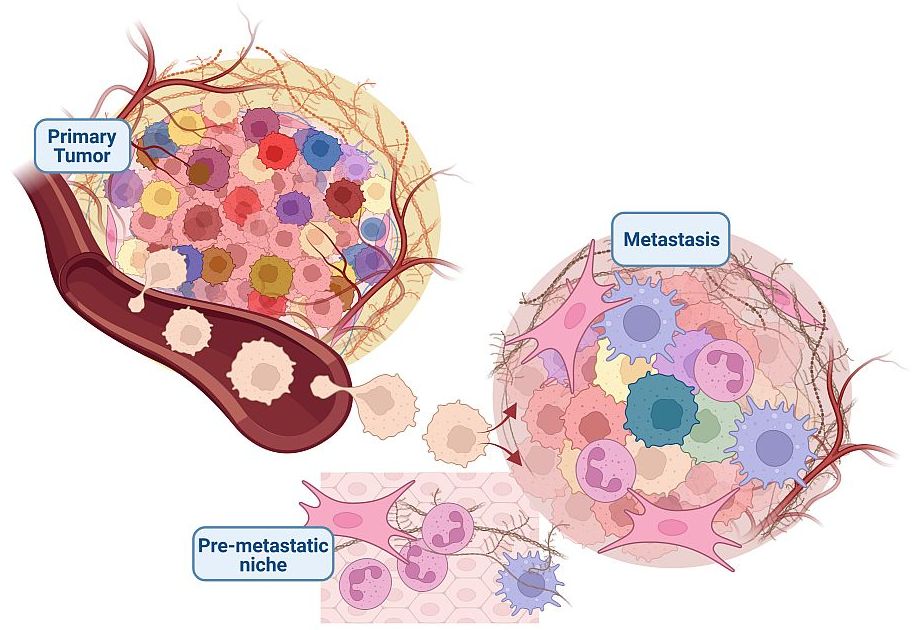
The main challenge in oncology remains the effective treatment of metastasis.
Current cancer treatment is most effective in attacking the primary tumor but has little effect on metastatic cells. This is a substantial problem because metastases account for the vast majority of cancer-related deaths. During the multistep process of metastasis, tumor cells adapt to various microenvironments that are distinct from their site of origin, but our understanding of the processes that lead to these adaptations is limited. Moreover, phenotypic alterations of metastatic cells may also cause resistance to therapeutics that cannot be accounted for by genotypic changes alone.
So why do some patients develop metastasis while others do not? Tumors consist of individual tumor cells that can vary in their genetic modifications (genetic intra-tumoral heterogeneity) as well as display very different phenotypes (phenotypic intra-tumoral heterogeneity). The individual tumor cells interact with their environment including a variety of non-malignant stromal and immune cells in a unique way that impacts their ability to metastasize.
The Lab of Tumor Heterogeneity and Metastasis is focused on achieving a better understanding of how tumor heterogeneity impacts metastatic behavior, how individual tumor cells interact with the microenvironment (Winkler et al. Nature Communications 2020), and in particular, how the innate immune system modulates (pre-) metastatic niches in distant organ sites.
We are using technology-driven systems-oncology approaches that lead to meaningful insights into the complex biology of metastasis. We co-developed MULTI-Seq (McGinnis et al. Nature Methods 2019), a novel multiplexing approach for single-cell RNA-sequencing (scRNA-Seq), which enables a cost-effective increase in sample size, reduction of batch effects, and the recovery of challenging cell populations.
In the Winkler Lab, we apply this scRNA-Seq technology together with other single-cell omics and spatial applications to understand the implications of tumor heterogeneity on:
- the formation and progression of metastasis
- the tumor-immune cell axis
- the therapy resistance of metastasis.
Selected Publications
Single-cell analysis of breast cancer metastasis reveals epithelial-mesenchymal plasticity signatures associated with poor outcomes
Winkler J#, Tan W, Diadhiou CMM, McGinnis CS, Abbasi A, Hasnain S, Durney S, Atamaniuc E, Superville D, Awni L, Lee JV, Hinrichs JH, Wagner PS, Singh N, Hein MY, Borja M, Detweiler A, Liu SY, Nanjaraj A, Sitarama V, Rugo HS, Neff N, Gartner ZJ, Pisco AO#, Goga A#, Darmanis S# & Werb Z.
# corresponding author
J. Clin. Invest. (2024) 134, e164227. doi: 10.1172/JCI164227
The temporal progression of lung immune remodeling during breast cancer metastasis
McGinnis CS, Miao Z, Superville D, Yao W, Goga A, Reticker-Flynn NE, Winkler J*#, Satpathy AT*#
# corresponding author * equal contribution
Cancer Cell (2024) 42(6), 1018-1031.e6. doi:10.1016/j.ccell.2024.05.004
The Tabula Sapiens: A multiple-organ, single-cell transcriptomic atlas of humans
Tabula Sapiens Consortium*
*Winkler J (Tabula Sapiens Consortium member)
Science. 2022 May 13;376(6594):eabl4896. doi: 10.1126/science.abl4896.
Bisphenol A replacement chemicals, BPF and BPS, induce pro-tumorigenic changes in human mammary gland organoid morphology and proteome
Winkler J#, Liu P, Phong K, Hinrichs JH, Ataii N, Williams K, Hadler-Olsen E, Samson S, Gartner ZJ, Fisher S#, Werb Z.
# corresponding author
Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2115308119. doi: 10.1073/pnas.2115308119.
Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis
Winkler J#, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z.
# corresponding author
Nat Commun 2020 Oct 9;11(1):5120. doi: 10.1038/s41467-020-18794-x.
MULTI-seq: sample multiplexing for single-cell RNA sequencing using lipid-tagged indices
McGinnis CS, Patterson DM, Winkler J, Conrad DN, Hein MY, Srivastava V, Hu JL, Murrow LM, Weissman JS, Werb Z, Gartner Z.
Nat Methods. 2019 Jul;16(7):619-626. doi: 10.1038/s41592-019-0433-8.
Alle Publikationen
Financial Support
| 01.09.2025 | |
| 25.07.2025 | |
| 06.05.2025 | |
| 25.07.2024 | Kurier TV: Krebsforschung: Wie KI bei Diagnose und Therapie helfen kann |
| 19.07.2024 | |
| 12.07.2024 | Dynamische Veränderungen im Immunsystem der Lunge bei der Metastasierung von Brustkrebs |
| 06.05.2024 | Universimed: Heureka! |
| 20.12.2023 | Juliane Winkler beim WWTF Life Sciences 2023 Call "Understanding Biology with AI/ML" erfolgreich |
| 18.08.2023 | Dr. Juliane Winkler ausgezeichnet mit der Fellinger Krebsforschungsförderung |
Bibliography
Dr. Juliane Winkler is a trained Pharmacist and received her Ph.D. from the University of Heidelberg (jointly EMBL, German Cancer Research Center, and Institute of Pathology), where she studied how nuclear transport alterations promote aggressive hepatocellular carcinoma (HCC). In her postdoc at the University of California San Francisco, Dr. Winkler worked with the late Zena Werb and Andrei Goga on the impact of tumor heterogeneity on breast cancer metastasis. She received multiple awards for her research and mentoring (e.g. EMBO long-term Fellow, AACR Scholar-in-Training Award, Dean’s Award for Excellence in Mentoring, honorable mention). The motivation for her research comes from years of caring for cancer patients as a trained oncology consultant in the public pharmacy, and her desire to improve treatment options for patients with metastasis.