In the Winkler Lab, we are investigating the implications of tumor heterogeneity on metastases. To this end, we are using technology-driven systems-oncology approaches that lead to meaningful insights into the complex biology of metastasis. We apply single-cell omics and spatial applications to understand the implications of tumor heterogeneity:
- on the formation and progression of metastasis
- the tumor-immune cell axis
- and the therapy resistance of metastasis.
Impact of tumor heterogeneity on the progression of metastasis
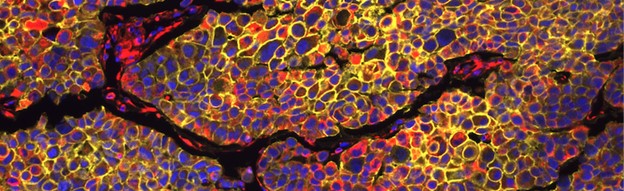
During the multistep process of metastasis, tumor cells adapt to various microenvironments that are distinct from their site of origin, but our understanding of the processes that lead to these adaptations is limited. Our lab applies different single-cell technologies to investigate the underlying mechanisms that lead to the metastatic behavior of individual tumor cells or cell states. One of these abilities is epithelial-mesenchymal plasticity (EMP) which allows cells to switch between epithelial and mesenchymal cell states. A better understanding of these processes will help to identify treatment strategies to prevent metastasis.
Impact of tumor heterogeneity on the remodeling of metastatic niches
Understanding the complex interplay between malignant cancer cells and the surrounding non-malignant stroma represents one of the major challenges in cancer research. How tumor heterogeneity influences systemic immune cell functionality, and how, in turn, immune cell phenotype impacts tumor cell plasticity and metastatic potential is poorly understood.
Besides their immune-suppressive phenotypes, emerging evidence shows that myeloid cells have direct pro-metastatic functions that are particularly important in establishing pre-metastatic and metastatic niches, thereby enabling successful colonization and outgrowth of metastatic cells (Winkler et al. Nature Communications 2020).
In our lab, we seek to understand the tumor-immune cell axis with regard to the pro- and anti-metastatic functions of myeloid cells. We investigate mechanisms on how myeloid cells influence metastatic colonization, support established metastasis and determine the mechanisms by which heterogeneous primary tumor and metastatic cells regulate these properties.
Impact of tumor heterogeneity on therapy resistance of metastasis
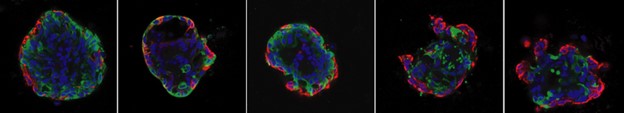
Current treatment is failing to eradicate metastatic disease which is likely promoted by the profound heterogeneity of tumor and metastatic cells and their plasticity.
Our lab is interested in how cancer treatment affects tumor heterogeneity and how tumor heterogeneity may promote therapy resistance. We apply organoid models of non-malignant and malignant tissues to test perturbations on cellular behavior (Winkler et al. PNAS 2022).
Using a rich single-cell readout we can map how individual tumor and metastatic cells respond to single therapeutics, a combination of therapeutics, or other perturbations.
With this strategy, we will identify and characterize so-called persister cells that withstand therapy and develop strategies to better treat metastasis.
