Therapy of late stage cancer
Besides immunotherapy, chemotherapy and therapy with small, targeted molecules are the two major strategies for the treatment of late stage cancer. In the past few decades, many new compounds have been developed and, consequently, improved therapy effectiveness.
However, even when using new, targeted drugs, therapy is often limited by strong side effects, resistance development and insufficient tumor delivery. Consequently, our research is focused on the development of new strategies to overcome these problems:
Investigation of the mechanisms underlying the sensitivity/resistance of cancer cells against anticancer drugs
Example: Thiosemicarbazones
With the aim of combating the strong iron dependency of cancer cells, various chelators have been developed in recent decades . Most prominent among these are thiosemicarbazones such as Triapine (currently in Phase III clinical development).
Our research aims to understand the mechanisms underlying thiosemicarbazone resistance (Miklos et al. Cancer Letters 2015, Bormio Nunes & Hager et al. Journal of Medicinal Chemistry 2020).
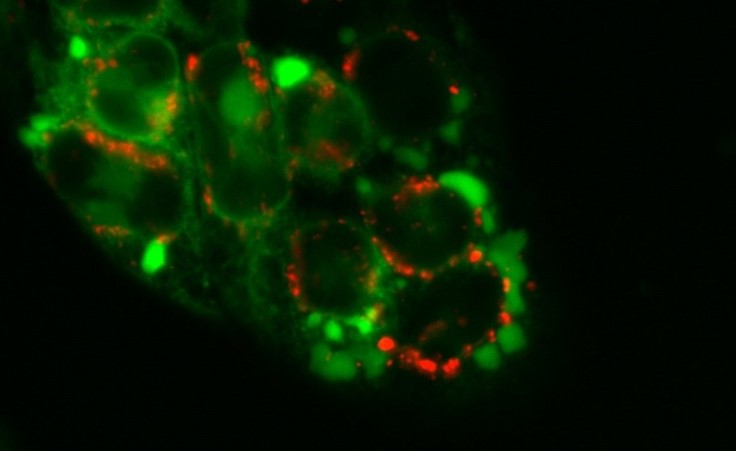
Furthermore, we (together with the team of Prof. C. Kowol) are intensively developing novel derivatives with higher tumor selectivity and activity (e.g. Kowol et al. J. Med. Chem 2016, Hager et al Antioxid Redox Signal. 2020) or are looking for formulations to improve the pharmacological properties of thiosemicarbazones (Mathuber & Hager et al Dalton Trans 2021). Of particular interest here is the property of some derivatives to trigger a new form of programmed cell death (paraptosis), which could be used to circumvent resistance to conventional cell death (apoptosis).
(Figure 1, Hager et al Cell Death Dis. 2018).
Development of new targeting strategies to increase drug delivery to the tumor tissue
In cooperation with the Institute for Inorganic Chemistry, University of Vienna as part of the "Research PlatformTranslational Cancer Therapy Research". The research platform was founded under the lead of Prof. B. K. Keppler and Prof. W. Berger with the aim of developing novel, better tolerable therapeutics (http://tctr.univie.ac.at/).
Our efforts are not only focused on the development of new drugs (e.g. KP1339, which is currently in phase II clinical trials), but also in particular on the design of prodrug concepts to improve already available therapeutics. In addition to receptor tyrosine kinase inhibitors, the focus here is on platinum compounds. New nanocarriers are also being tested.
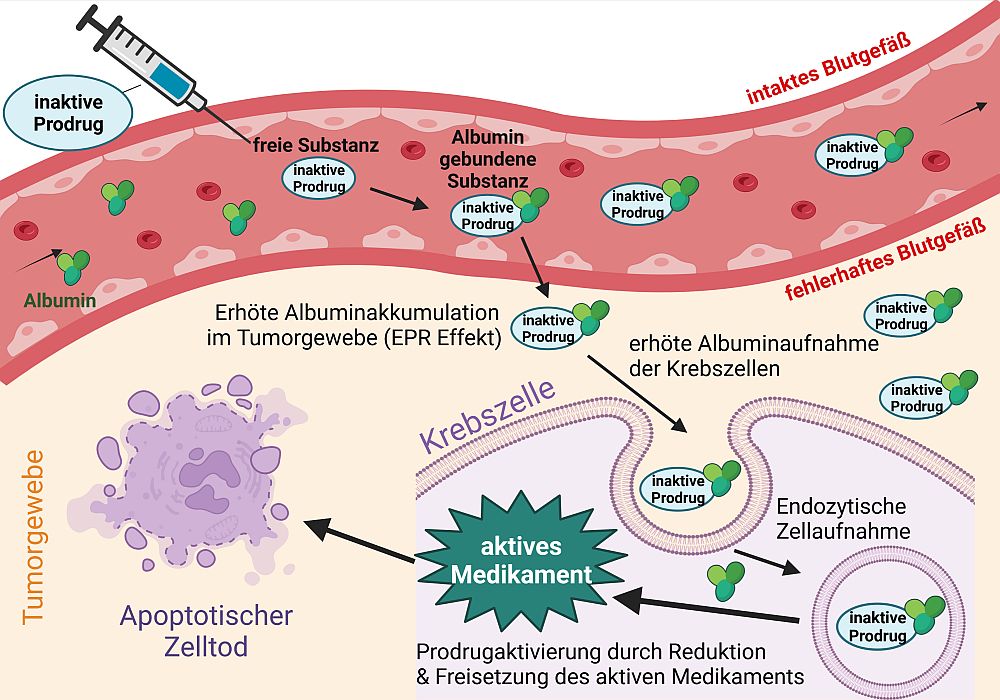
In addition to the first EGFR inhibitor that can be activated by hypoxia (Karnthaler-Benbakka et al. Angewandte Chemie 2014), our greatest achievements (together with the team of Dr. C. Kowol) include the development of albumin-binding platinum prodrugs (Figure 2, Mayr et al Chemical Science. 2017, Schüffl & Theiner et al Chemical Science 2021).
Improvement of anticancer activity by smart drug combinations
In order to improve the effectiveness of cancer therapy, synergies in the effectiveness of two drugs can be used. Over the years we have discovered and scientifically studied several such synergies.
An example is the synergism between arsenic trioxide (ATO), one of the oldest known drugs successfully used against APL, with EGFR inhibitors (Kryeziu et al. Mol Cancer Ther. 2013).
In the case of platinum compounds, the combination with immune stimulators has proven particularly promising (Groza et al. Oncoimmunology 2018).
If synergies are discovered, we can, together with our cooperation partners from the Institute of Inorganic Chemistry, produce intelligent molecules that release both active substances simultaneously in the malignant tissue (e.g. Fronik & Poetsch Journal of Medicinal Chemistry 2021, Karmakar & Poetsch Inorganic Chemistry 2019).