Stationary cells
Replication-independent mutations - mutations that occur in stationary cells - bear particular importance. Since they may lead to a hidden accumulation of damages, they play a crucial role in cancerogenesis and gain importance in old age, when we are faced with an increased genomic instability, e.g. NHEJ exhibiting a significantly elevated error ratio.
By studying these mechanisms, using the model organism S. cerevisiae, we hope to learn about the interrelations that in the long run may lead to the loss of growth control and to genome instability (which are typical properties of tumor cells).
Oxidative stress
Reactive oxygen species (ROS) are generated during normal cellular metabolism. Increased levels of ROS occur as a result of different kinds of stress. ROS may damage essential cellular macromolecules (lipids, proteins, nucleic acids) and are suspected to play a role in the development of several human diseases, ageing, and cancer.
The mutagenicity of oxidative DNA damage in proliferating cells is well investigated. For example it is known, that the quantitative most important oxidative DNA damage - the change of guanine to 8-oxo-guanine - finally leads to a transversion mutation from G to T (since during replication most likely a dAMP will be integrated opposite to the 8-oxo-guanine). However, it is quite unclear in which ways ROS cause mutations in quiescent, non-proliferating cells (as the majority of somatic cells in adults represent).
Using the model organism S. cerevisiae, we investigate how intracellular ROS may generate mutations in resting cells; e.g. via error prone DNA repair mechanisms.
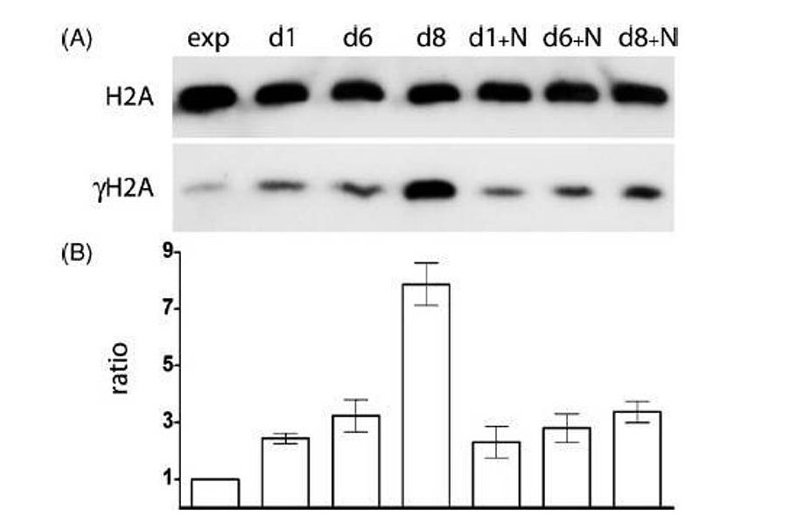
DNA double strand break-repair
DNA double strand breaks (DSBs) can be caused by ionizing radiation, oxygen radicals, and several chemicals. They are a severe threat for cells and are a potential reason for cancer. Furthermore, it is likely that the accumulation of unrepaired DSBs is one of the reasons of the ageing process of mammalian cells.
Repair of DSBs is essential for the maintenance of genomic integrity and the survival of cells. Three major repair pathways are known: homologous recombination (HR), non-homologous end joining (NHEJ), and single-strand annealing (SSA).
We investigate these pathways using the model organism Saccharomyces cerevisiae. HR is an error-free repair mechanism in which the rebuilding is carried out according to an existing intact sequence, that is typically located at the sister chromatid or at a homologous chromosome. SSA does not need homologous chromosomes, but adjacent homologous sequences at both sides of the DSB. Presented terminally, they will be joined and finally ligated covalently. The repair of DSBs via NHEJ occurs by ligating the loose ends regardless of any template.
All three pathways participate in the DSB in mammalian cells. The involvement of the different pathways changes according to cell-cycle state, ploidy of the cells, and probably some other - yet unknown - influences.