PLASMAX: Less Artefacts, More Tumour-relevant Cell Cultures
To study cancer cells in more physiologically-relevant conditions, my group developed Plasmax™, a chemically-defined cell culture medium based on the levels of metabolites present in human plasma (Vande Voorde et al. Science Adv. 2019).
We are convinced that implementing physiological culture conditions will predict more reliably in vivo physiology and consequently the biology of tumours. We have recently performed a whole-genome CRISPR-based screen comparing gene essentiality in triple negative breast cancer (TNBC) cells cultured in DMEM and in Plasmax™ (in collaboration with the CRUK-Astra Zeneca Functional Genomic Centre, Cambridge, UK). We are currently testing the relevance of the identified medium-specific essential metabolic genes for TNBC.
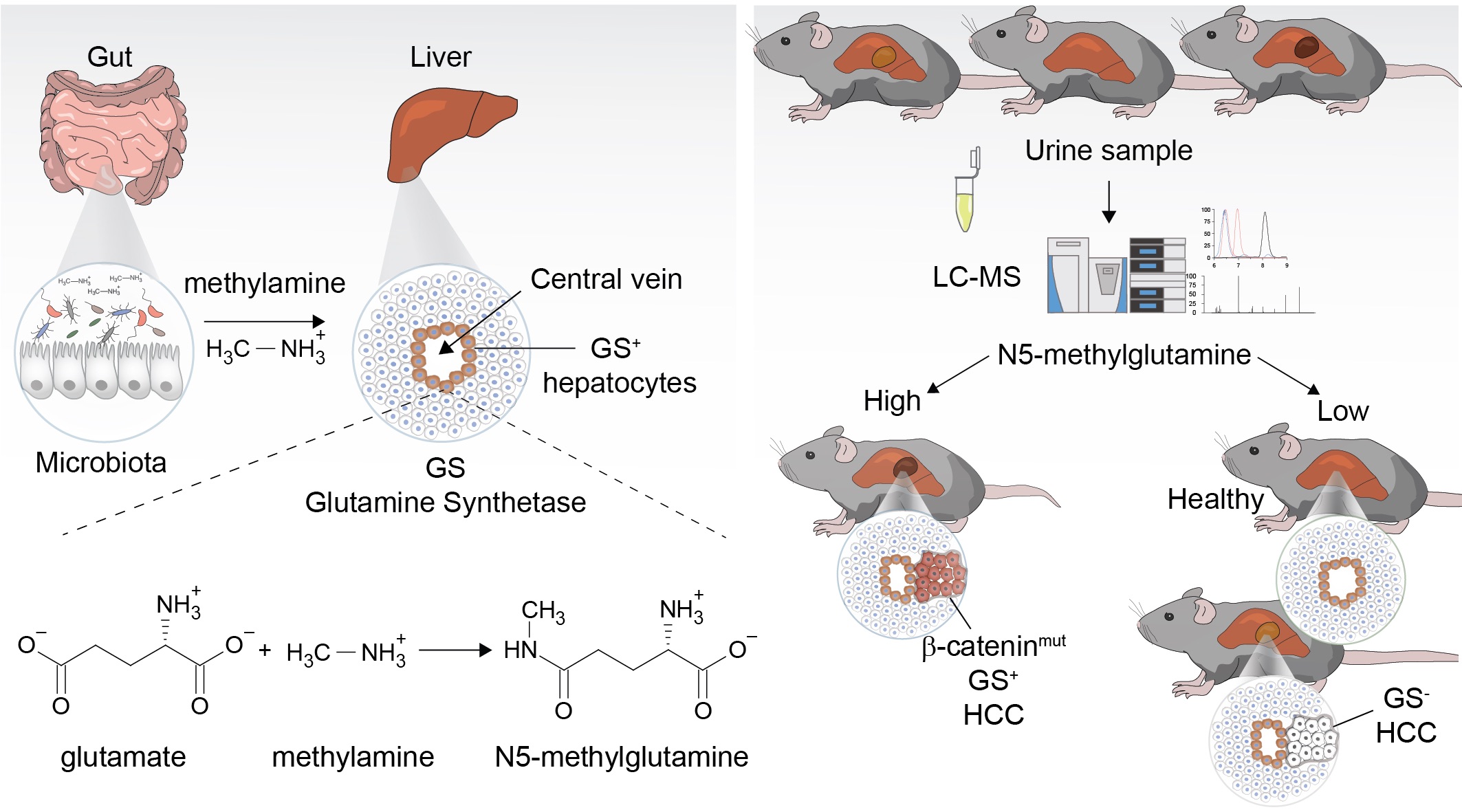
The Role Of Glutamine Metabolism In Liver Physiology And Cancer
We discovered N5-Methylglutamine as a novel product of glutamine synthetase, adding new connections to the interorgan-microbiome metabolic network. Moreover, N5-methylglutamine levels in urine accurately predicts HCC tumour burden in murine models, exemplifying the translational potential of our metabolic discovery science (Villar et al. Nat Chem Biol 2022). To assess the clinical validity of N5-methylglutamine as non-invasive stratification marker, we will analyse plasma and urine samples from a cohort of HCC patients enrolled in a dedicated clinical trial (clinical lead Dr Tom Bird, University of Edinburgh). The aim is to stratify patients with b-catenin driven HCC aiding personalized treatment. This translational project has been funded by the Sir Jules Thorn Trust UK.
Lipid And Selenium Metabolism Govern Ferroptosis In Triple Negative Breast Cancer (TNBC)
Ferroptosis is a type of cell death caused by iron-dependent lipid peroxidation counteracted by the antioxidant activity of selenoproteins. We have found that TNBC cells grown at high density in culture or as mammary tumours secrete monounsaturated fatty acids (MUFAs) that prevent ferroptosis.
However, circulating TNBC cells decrease the expression of MUFA-producing enzymes and become particularly sensitive to the inhibition of selenocysteine biosynthesis that prevent their lung seeding (Akermann et al. EMBO Mol Med 2024). The development of compounds that inhibit selenocysteine biosynthesis offers a promising new strategy to target TNBC metastasis by exploiting the vulnerability of circulating tumour cells to ferroptosis, potentially improving patient outcomes in this aggressive cancer subtype.
Glioblastoma Metabolism On Steroids
Dexamethasone, a steroidal anti-inflammatory drug, is frequently used to manage brain tumour patients. We found that dexamethasone has a direct impact on glioblastoma metabolism diverting methyl groups to 1-methylnicotinamide via Nicotinamide N-Methyltransferase (NNMT), as shown by stable isotope tracing of nicotinamide in glioblastoma patients. We exploited the tumour-specific NNMT activity to visualize brain tumours with a novel nicotinamide PET tracer and identified a dietary intervention that has synergistic therapeutic effects if coupled with dexamethasone.
Glutaminase Inhibition In Braf-Mutant Melanoma
BRAF/MEK inhibitors are first line treatments for BRAF mutant melanoma that rewire tumour metabolism. We found that in vivo, acute, but not long-term BRAF inhibition sensitizes BRAF-mutant melanoma to CB839, a clinically-safe glutaminase inhibitor. Further investigation into the metabolic vulnerabilities induced by MEK inhibition and glutaminase blockade will lead to rationally-designed therapeutic combinations for improving outcomes in BRAF-mutant melanoma.