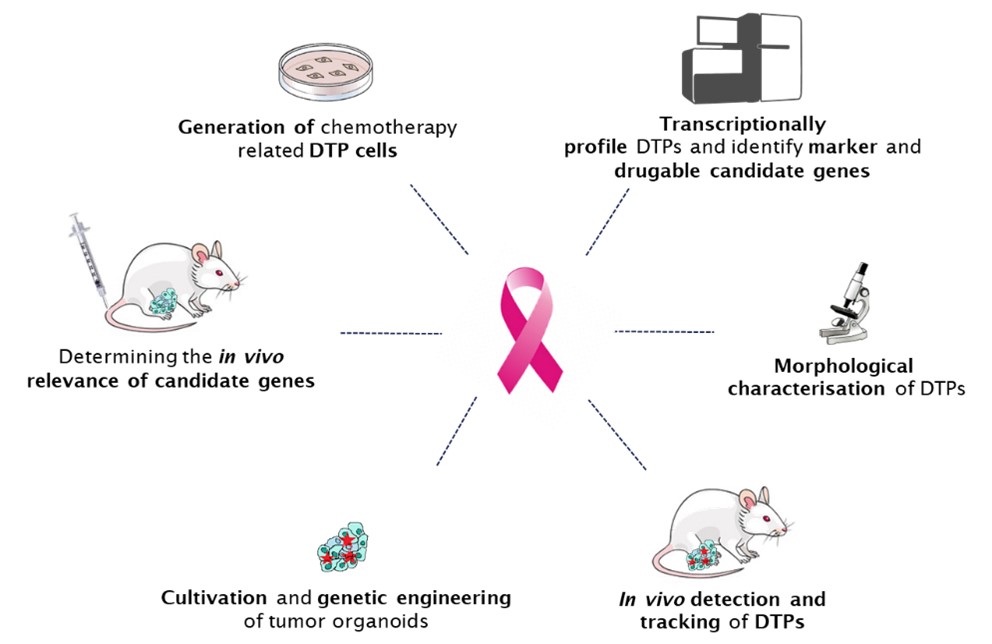
General concept
Our preliminary work has characterized the response of orthotopically transplanted tumor-bearing mice to a series of clinically relevant chemotherapies. While these therapies significantly reduce the tumor size, they are unable to eradicate tumor cells, which give rise to drug-sensitive relapse after the cessation of the treatment.
We address the following research questions:
- What is the contribution of phenotypic adaptation vs Darwinian selection?
- Are cells showing a DTP signature present prior to treatment?
- Will different clinical protocols induce DTPs with similar signatures?
- What is the relative contribution of genetic vs non-genetic (epigenetic, transcriptional plasticity) factors, and of the interactions with the microenvironment?
- What is the relation between the pathways supporting DTPs and the mechanisms underlying resistance to therapy?
- How can this knowledge be translated to improved patient care?
To address these challenging questions, we will profile individual “tumor histories” at single-cell resolution using in vitro cell lines and organoids derived from genetically engineered mouse models of cancer (GEMMC).
1. Targeting Drug-tolerant Persisters: a paradigm shift to conquer therapy resistant breast cancer
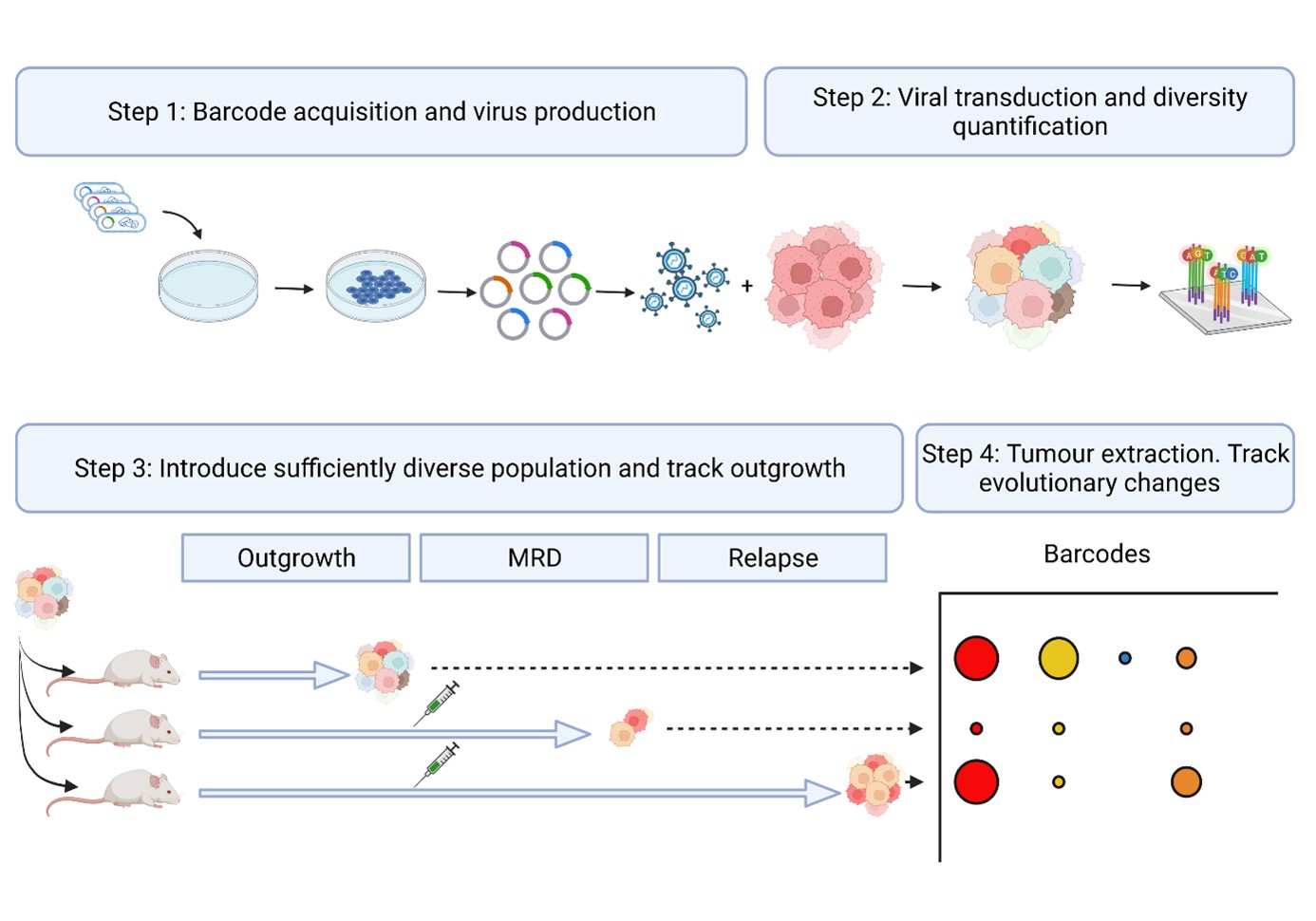
We will establish, treat and sample barcoded mammary tumors derived from organoids to track (epi)genetic and transcriptional alterations along the treatment. Matched samples corresponding to treatment-naïve cells, DTPs and relapse will be collected. In experiments aimed at obtaining therapy resistant tumors, chemotherapy will be continued until treatment becomes ineffective.
2. Analysis of pathways sustaining drug tolerance using organoid based breast cancer models
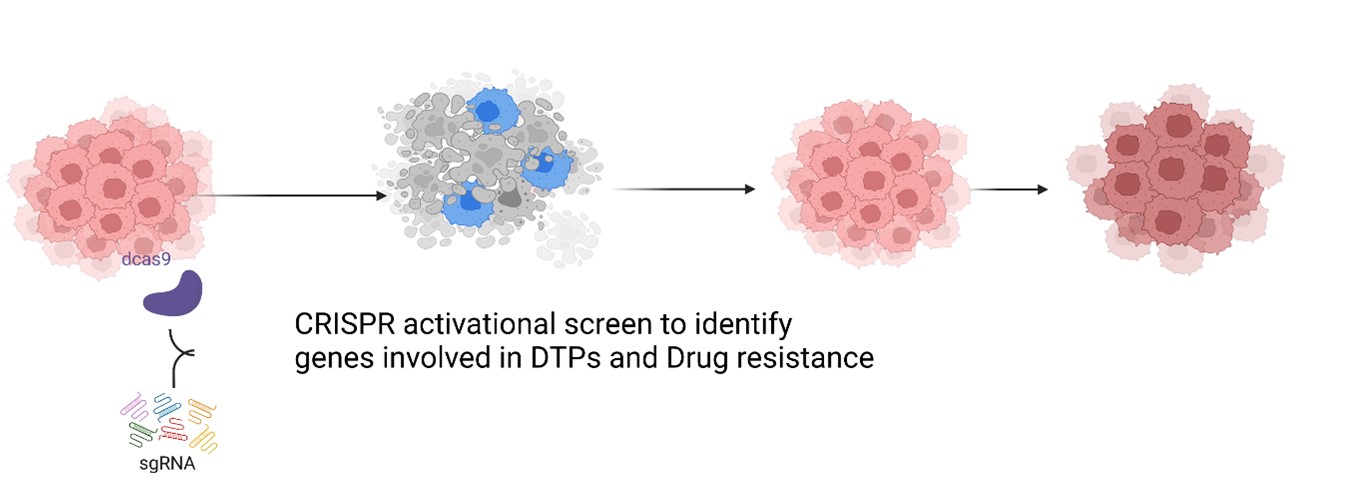
The main aim is to identify genes of therapeutic relevance involved in DTP survival. We will perform an unbiased genome-wide CRISPR-SAM activation screen using a 2D in-vitro repopulation assay established earlier in the lab. We will validate the relevance of candidate genes using 3D tumour organoid and genetically engineered mouse models (GEMM).
3. REAP: Revealing drug tolerant persister cells in cancer using contrast enhanced optical coherence and photoacoustic tomography
In REAP we envision to provide imaging systems useful across multiple scales to reveal drug tolerant persister cells in a preclinical setting. We will develop a triple modal two-photon laser scanning – optical coherence – photoacoustic microscopy for in vitro investigations as well as a dual modal optical coherence photoacoustic tomography for in vivo applications. Parallel developments in contrast agents based on biofunctionalized nanoparticles, new lasers, detector technology and real-time data handling aided by deep learning-based analysis will lead to the detection, characterization, and eventual eradication of persister cells.
4. Identification of novel driver genes of chemoresistance
The main goal of this project is to use in vitro and in vivo assays to identify and characterise elusive drug-tolerant persister cells. Using an established in vitro repopulation assay to model cancer recurrence after chemotherapy in vitro, we will profile treatment-naïve, DTP and “relapsed” cells by RNA sequencing.
5. Uncovering the role of extracellular matrix in resistance to EGFR inhibitor treatment in triple negative breast cancer
Our aim is to uncover the role of the extracellular matrix (ECM) in therapy resistance, by utilising mouse models, clinically relevant treatments, and complex in vitro models. It is our hope to identify specific ECM components that can be targeted in order to sensitize TNBC patients towards therapy.