Molecular epidemiology of colorectal cancer
Colorectal cancer (CRC) is the third leading cancer related cause of death worldwide and represents a major public health problem. Incidence rates in Austria, with about 4,500 new CRC cases diagnosed in 2022, rank within the lower third within the EU.
The natural history of CRC usually involves slow progression from precancerous polyps to cancer, which offers opportunities for screening and early detection. Early detection of CRC is an important issue since stage at diagnosis remains the most important prognostic factor for CRC.
Genome-wide association studies of CRC
CRC is a complex disease with both genetic and lifestyle factors contributing to individual risk. Up to 35% of inter-individual variability in CRC risk has been attributed to genetic factors. In the last decade genome-wide association studies (GWAS) have identified over 250 risk loci for sporadic CRC. However, only a fraction of all CRC risk loci has been identified thus far and there is still missing heritability.
The "Molecular epidemiology research group" has already performed CRC GWAS, using Axiom arrays (Affymetrix), comprising 1.060 CRC, 689 high risk adenomas and 4.367 controls using the biobank "Colorectal Cancer Study of Austria" (CORSA). We pursued a dual approach, single marker analysis as well as model selection, to investigate genome-wide associations with CRC risk (Hofer et al. 2017).
Because of the importance of large-scale GWAS collaboration, we have integrated our GWAS data into large CRC consortia such as "Genetics and Epidemiology of Colorectal Cancer Consortium" (GECCO) and "COlorectal cancer GENeTics" (COGENT) to expand the catalog of CRC risk loci and to provide further insights into CRC susceptibility.
The identification of inherited genetic factors involved in CRC susceptibility can help to profile individual risk and may enable personalized screening decisions. CRC genetic susceptibility variants are thus likely to interact with environmental risk factors and they will be incorporated into models of predisposition.
Metabolomic profiles throughout the continuum of colorectal carcinogenesis (MetaboCCC)
Metabolomics is an innovative and powerful approach by which a large number of metabolites are systematically screened to characterize biological phenotypes with an unprecedented level of precision. New biomarkers that help characterize risk of progression along the pathway of colorectal carcinogenesis are urgently needed to tailor prevention strategies.
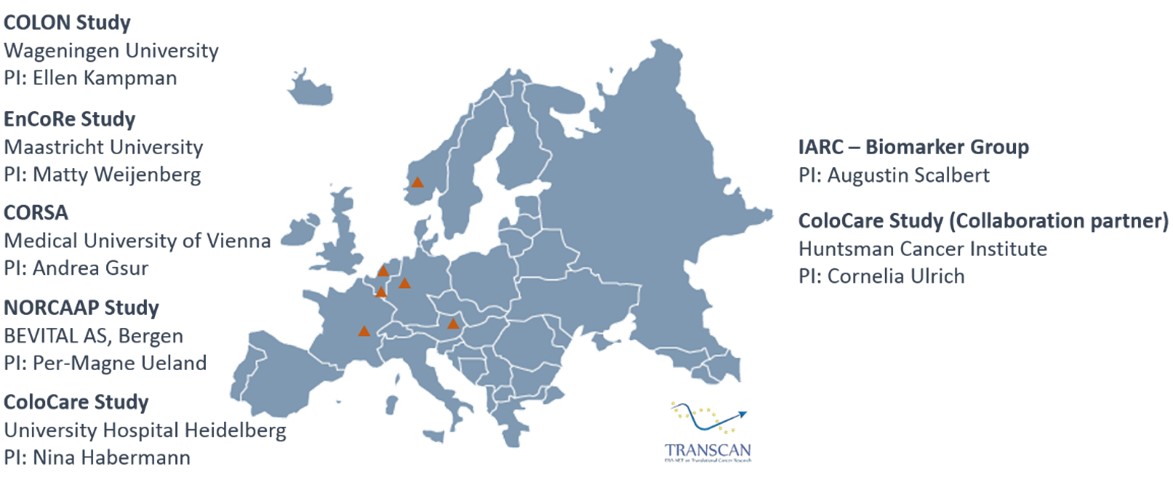
By investigating plasma from individuals free of colorectal tumors, patients with colorectal adenoma, and patients with CRC (stage I-IV), the MetaboCCC Consortium aims to investigate changes in the metabolome along the continuum of colorectal carcinogenesis. We will perform metabolomic analyses in 2.300 plasma samples derived from well-defined populations of four TRANSCAN countries (The Netherlands, Germany, Austria, Norway) with assays completed at an expert site at the International Agency for Research on Cancer (IARC, France), using multiple discovery and replication sets to define biologic mechanisms of colorectal carcinogenesis. For a comprehensive and complementary strategy, we will apply both targeted and untargeted metabolomics.
We expect that the discovery of novel metabolites in blood that define the transition between various stages of colorectal carcinogenesis can be used in the future for risk stratification, including for tailored prevention strategies by endoscopy.
Biomarkers related to folate-dependent one-carbon metabolism in colorectal cancer recurrence and survival (FOCUS)
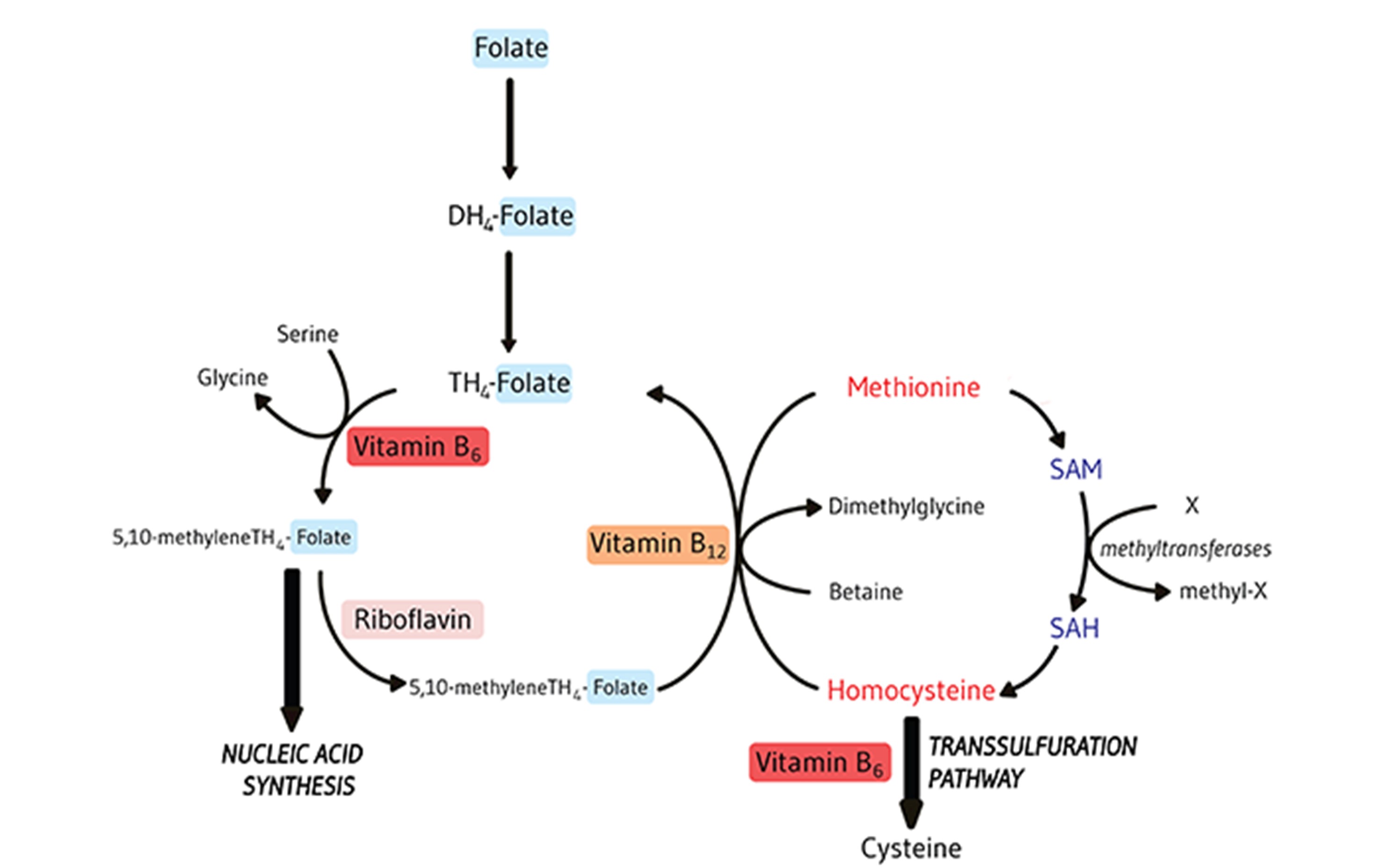
CRC is the third leading cause of cancer death. At the same time, the number of survivors of CRC continues to increase. Many cancer patients resort to taking nutritional supplements, assuming potential benefits. However, in the case of folate this assumption may be wrong since high folate may stimulate the growth of established cancer. Folate is involved in one-carbon metabolism, impacts DNA synthesis, and supports tumor growth.
Within a large consortium of parallel European CRC patient cohorts, we are able to investigate, comprehensively, the role of folate from diet and supplements, the impact of one-carbon metabolism biomarkers, and their joint influence on clinical outcomes.
Our study aims to investigate whether prognosis in CRC is related to folate status at different time-intervals in 1.584 CRC patients. Both, key biomarkers of folate-mediated one-carbon metabolism (FOCM) and diet/supplements will be investigated as components of folate status prior to surgery, and at 6 and 12 months past surgery. Further, we will determine whether biomarkers related to FOCM are associated with folate intake and explore whether folate status modifies treatment toxicity in patients who underwent 5-FU chemotherapy.
Gut MICRObiome-based approach for incorporating new biomarkers into COLOrectal cancer screening (MICROCOLO)
There is an urgent demand for novel non-invasive screening methods to identify individuals who should undergo colonoscopy and those who need colonoscopy at an earlier age than recommended in most screening programs. The gut microbiota is believed to be directly involved in colorectal carcinogenesis. There is evidence that changes in the gut microbiome occur during different stages of colorectal neoplasia, supporting an etiologic and diagnostic role for the microbiome. Harnessing knowledge of the microbiota may lead to new preventive strategies and diagnostics.
Integration of the microbiome study into the province-wide CRC screening program "Burgenland Prevention Trial of Colorectal Cancer Disease with Immunological Testing" (B-PREDICT) based on Fecal Immunochemical Tests (FIT) enables the collection of a large number of FIT samples for secondary use for microbiome analysis.
myBioma GmbH - our company partner within this FFG Bridge funded project - will use their already established data processing and statistical analysis workflow to process the resulting microbiome data. Predictive microbiome-based signatures specific for CRC and high-risk adenomas will be defined by multivariate statistical modelling. The combination of conventional screening methods such as FIT with microbiome-based methods is a promising tool for early detection of CRC and could improve diagnostic accuracy.